Abstract
Background:
Graft versus Host Disease (GVHD) is a common complication of allogeneic hematopoietic stem cell transplant (HSCT). In this condition, the donated stem cells attack the recipient cells, leading to life-threatening consequences. One of the methods of preventing GVHD is by using
post-transplantation cyclophosphamide (PTCy). PTCy has proven to have minimum toxicities to the body and, at the same time, has a high success rate in mitigating the rate of rejection and reducing the incidence of GVHD by acting as a potent immunosuppressant on both B and T cell lymphocytes in the host and donor's cells. This systematic review and meta-analysis was conducted to observe the effect of using PTCy for GVHD prophylaxis after HSCT.
Methodology:
Following Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines, a comprehensive literature search was conducted on PubMed, Cochrane Library, and ClinicalTrials.gov through September 2021. We used MeSH terms and keywords for
"Cyclophosphamide" AND "stem cell transplantation" AND "GVHD." No filters or publication time limits were applied to the search. A total of 17 studies were included after the screening of 1145 records and excluding duplicates, reviews, and non-relevant articles. Quality evaluation was done using the NIH quality assessment tool, and inter-study variance was calculated using Der Simonian-Laird Estimator. Pooled analysis was done using the 'meta' package (Schwarzer et al.R programming language) and proportions with 95% confidence intervals (CI) were computed.
Results:
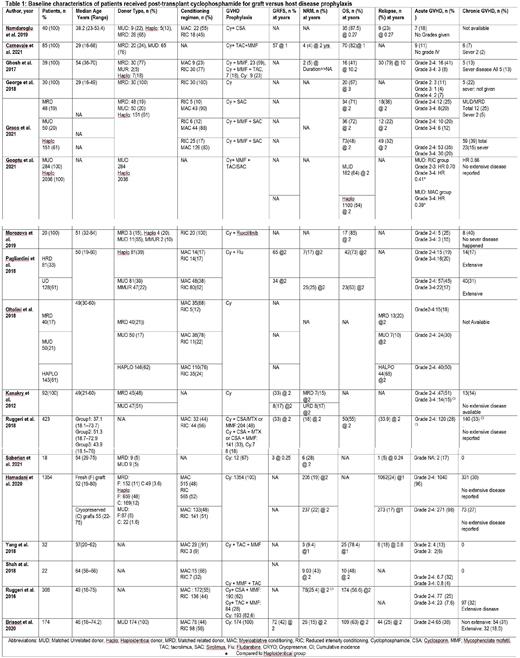
A total of 5649 patients from 17 studies were included in this meta-analysis. (Table 1) The median age of patients was 49 (18-80) years, and 24.8% (1402/5649) were males. The median duration of follow-up was 23 (1-158.4) months. The donor type was haploidentical 70%
(3258/4650), matched unrelated donor (MUD) 16% (761/4650), matched related donor (MRD) 13% (580/4650), and mismatched unrelated donor (MMUR) 1% (49/4650). The conditioning was myeloablative (MAC) in 53% (1461/2734) and reduced intensity (RIC) in 47% (1273/2734) of the patients. The pooled median overall survival (OS) reported at two years was 59% (95%CI 0.53-0.64, I 2 =66%, p=0.02, n=2843) while the pooled relapse rate (RR) was 29% (95%CI 0.11-0.51, I 2 =92%, p <0.01, n=303). The pooled incidence of acute GvHD grade II-IV and grade III-IV was 26% (95%CI 0.19-0.35, I 2 =80%, p <0.01, n=859) and 12% (95%CI 0.04-0.32, I 2 =94%, p<0.01, n=562), respectively. The pooled incidence of chronic GVHD was 17% (95%CI 0.11-0.26, I 2 =90%, p<0.01, n=819) with pooled incidence of extensive chronic GVHD of 9% (95%CI 0.05-0.19, I 2 =83%, p<0.01, n=697) as reported by seven included studies. The pooled incidence for non-relapse mortality (NRM) was 16% (95%CI 0.09-0.24, I 2 =83%, p< 0.01, n=769).
Conclusion:
Post-transplant Cyclophosphamide is an effective treatment option for graft versus host disease prophylaxis after allogeneic hematopoietic stem cell transplantation with an acceptable toxicity profile. However, further randomized trials are needed to consolidate these findings.
Disclosures
Abhyankar:Therakos: Consultancy, Research Funding, Speakers Bureau; Incyte: Consultancy, Research Funding, Speakers Bureau. McGuirk:Novartis: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Speakers Bureau; Nextar: Consultancy, Honoraria; Orca Bio: Research Funding; Magenta Therapeutics: Consultancy, Honoraria, Research Funding; Allovir: Consultancy, Honoraria, Research Funding, Speakers Bureau; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; Sana: Honoraria; CRISPR Therapeutics: Consultancy; In8bio, Inc.: Other: IIT Clinical Trial.
Author notes
Asterisk with author names denotes non-ASH members.